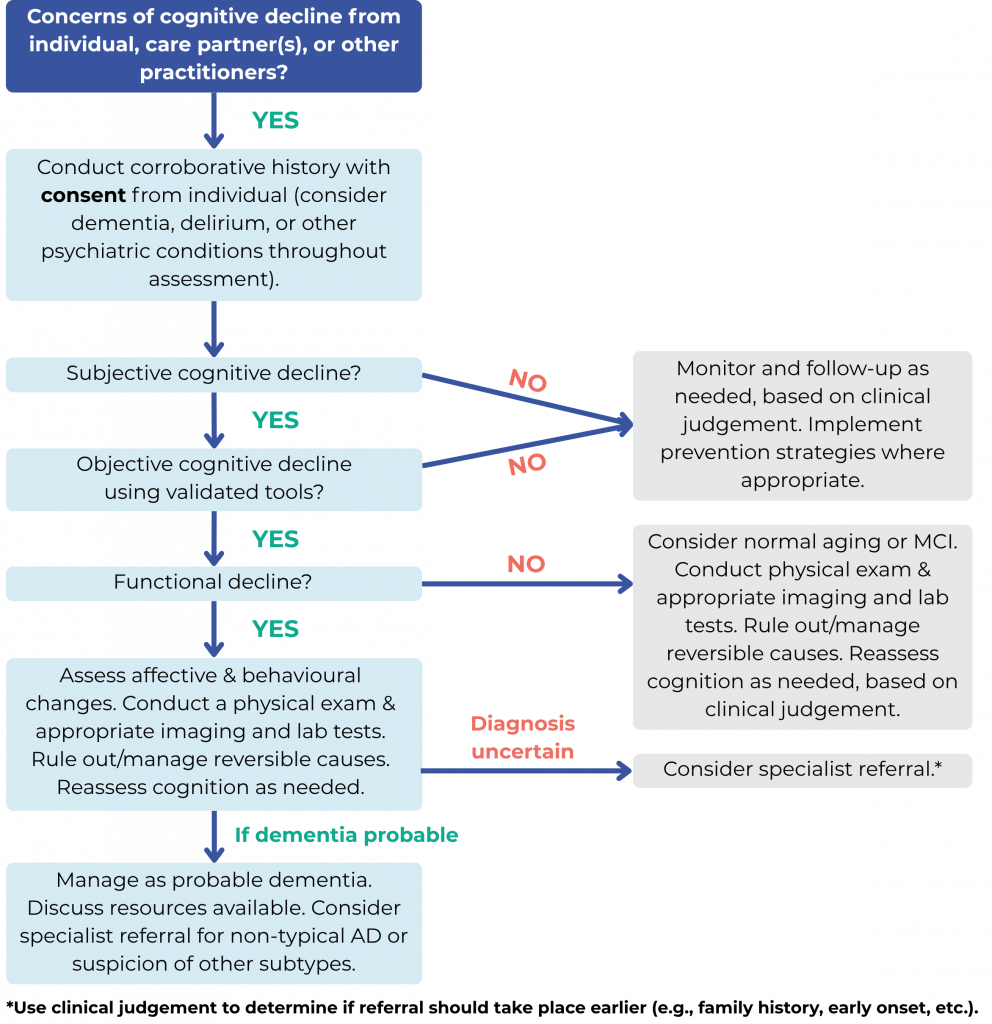
The Dementia Diagnosis tool was developed using the Centre for Effective Practice’s (CEP’s) integrated knowledge translation approach as part of the Knowledge Translation in Primary Care (KTinPC) Initiative. This approach ensures that clinicians are engaged throughout the development processes through the application of user-centered design methodology. Clinical leadership of the tool was provided by Drs. Andrea Moser and Sid Feldman. End users and clinical experts were also engaged to provide feedback. Funded by the Ministry of Health, the KTinPC Initiative supports primary care clinicians with a series of clinical tools and health information resources. Learn more about the KTinPC Initiative.
This Tool was developed for licensed health care professionals in Ontario as a guide only and does not constitute medical or other professional advice. Health care professionals are required to exercise their own clinical judgement in using this tool. Neither the CEP, Government of Ontario, nor any of their respective agents, appointees, directors, officers, employees, contractors, members or volunteers: (i) are providing medical, diagnostic or treatment services through this Tool; (ii) to the extent permitted by applicable law, accept any responsibility for the use or misuse of this Tool by any individual including, but not limited to, primary care providers or entity, including for any loss, damage or injury (including death) arising from or in connection with the use of this Tool, in whole or in part; or (iii) give or make any representation, warranty or endorsement of any external sources referenced in this Tool (whether specifically named or not) that are owned or operated by their parties, including any information or advice contained therein.
This Tool is a product of the CEP. Permission to use, copy, and distribute this material is for all noncommercial and research purposes is granted, provided the above disclaimer, this paragraph and the following paragraphs, and appropriate citations appear in all copies, modifications, and distributions. Use of this Tool for commercial purposes or any modifications of the Tool are subject to charge and must be negotiated with the CEP (info@cep.health).
For statistical and bibliographic purposes, please notify the CEP (info@cep.health) of any use or reprinting of the Tool. Please use the following citation when referencing the Tool: Reprinted with Permission from Centre for Effective Practice. (July 2025). Dementia Diagnosis: Ontario. Toronto: Centre for Effective Practice.