Pelvic organ prolapse (POP) is the herniation of the pelvic organs to or beyond the vaginal walls. Many women with POP experience symptoms that impact daily activities, sexual function, and exercise.14 The degree of POP doesn’t always co-relate with the degree of UI symptoms that a patient experiences.
Screening for POP
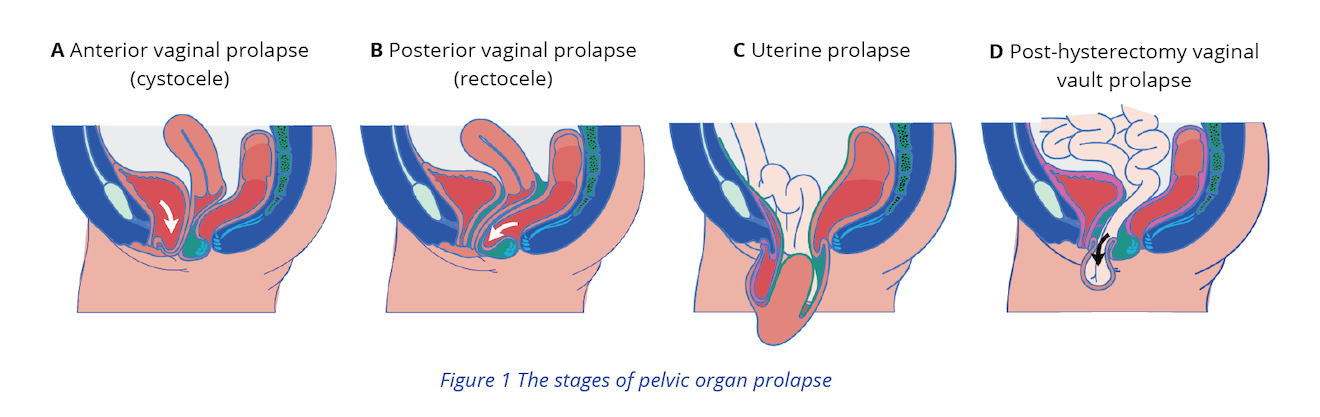
If examination of the perineum and assessment of pelvic floor muscles leads to suspected POP, treat POP concurrently with UI and proceed with the following steps:3
- After conducting the examination to detect signs of POP, use the Pelvic Organ Prolapse Quantitation (POP-Q) system to confirm signs of prolapse. POP-Q is an objective, site-specific system for describing and staging POP in women.13,15
- If a patient has symptoms of prolapse, not explained by a physical examination, consider repeating the examination at another scheduled appointment or with the woman in a standing or squatting position.6
- If patient with POP presents with any of the following symptoms, refer them to the appropriate specialist (gastroenterologist, gynaecologist or urologist):6,7
- Pain
- Symptoms of obstructed defecation or faecal incontinence
- Symptoms not explained by examination findings
Treatment options
Discuss management options with women who have pelvic organ prolapse, including no treatment, non-surgical treatment and surgical options, taking into account:6
- Patient’s preferences
- Site of prolapse
- Lifestyle factors
- Comorbidities, including cognitive or physical impairments
- Age
- Desire for childbearing
- Previous abdominal or pelvic floor surgery
- Benefits and risks of individual procedures
Management options
Non-Surgical options
- Lifestyle interventions
- Pelvic floor training
- Appropriate for: All patients if they can tolerate vaginal examinations.3,7
- How to: Consider referring patient to a pelvic floor physiotherapist/physiotherapist for supervised pelvic floor muscle training for at least 16 weeks as a first option for women with symptomatic POP-Q stage 1or stage 2 pelvic organ prolapse. If the program is beneficial, advise women to continue pelvic floor muscle training afterwards.6
- Consider referring patients to Patient Resources section for additional pelvic floor training support
- Pessaries
- Appropriate for: Patients with symptomatic pelvic organ prolapse
- How to: Obtain a pessary fitting kit. As wait times for pessary fitting and care may vary, providers can learn to do this either by themselves by referring to the Canadian Family Physician article on pessary fittings or by shadowing an expert in this area.3,48
- If the patient has vaginal odour, bleeding, or is post-menopausal, consider a low-dose of topical estrogen in conjunction with pessary use after clinical examination.50,51
- Refer patients with vaginal erosion and ongoing bleeding to a specialist.3
- Instruct the patient on pessary insertion/removal and care (cleaning and examination), as needed. Also ask about comfort and side effects.34
- Follow-up: Every 6 months with a gynaecological examination included
Surgical options
If your patient is considering surgical procedures for POP, use decision aids found in Patient resources to promote informed preference and shared decision making. Refer the patient to a specialist with an interest in incontinence if they choose elective surgery.3,32,33
Proceed to Diagnosis to treat UI symptoms concurrently with POP.

 Is pelvic organ prolapse suspected?
Is pelvic organ prolapse suspected?
