SP drafting
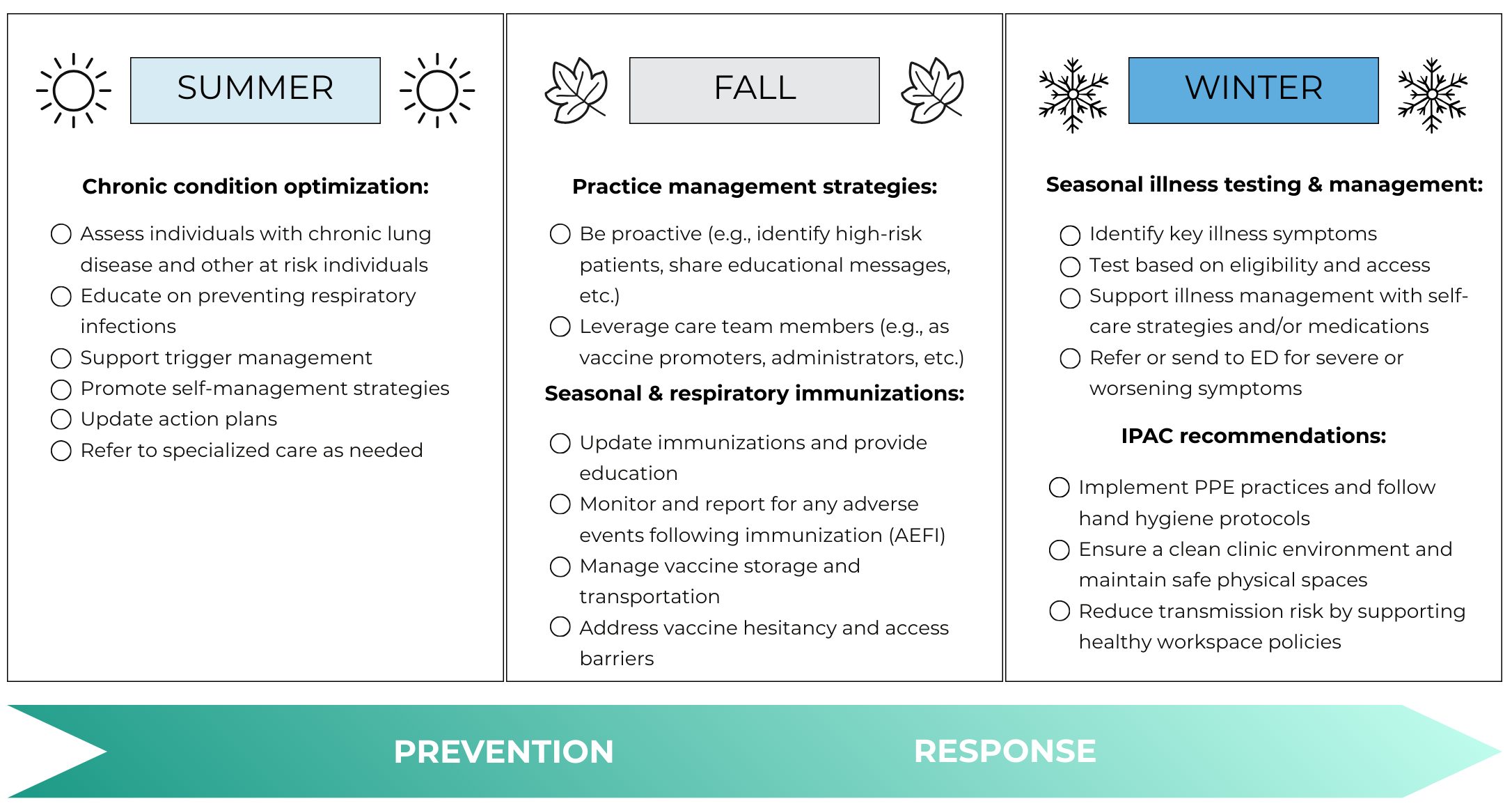
The following image provides a high-level summary of key seasonal preparedness activities. For more information, explore the detailed content below.
Seasonal illness testing and management (coming this winter)
References
-
[1]
Asthma Canada. Be Winter Ready – Tips for Managing Your Asthma This Season [Internet]. 2025 [cited 2025 Jul 9]. Available from: https://asthma.ca/be-winter-ready-tips-for-managing-your-asthma-this-season/
-
[2]
CDC. Living with Asthma [Internet]. Asthma. 2024 [cited 2025 Jul 9]. Available from: https://www.cdc.gov/asthma/living-with/index.html
-
[3]
CDC. Respiratory Infections and Asthma [Internet]. Asthma. 2024 [cited 2025 Jul 9]. Available from: https://www.cdc.gov/asthma/respiratory-infections/index.html
-
[4]
Yang CL, Hicks EA, Mitchell P, Reisman J, Podgers D, Hayward KM, et al. 2021 Canadian Thoracic Society Guideline – A focused update on the management of very mild and mild asthma. Can J Respir Crit Care Sleep Med. 2021;5(4):205–45.
-
[5]
Bourbeau J, Bourbeau M, Hernandez P, Hernandez S, Beauchesne M, Kermelly S, et al. 2023 Canadian Thoracic Society Guideline on Pharmacotherapy in Patients With Stable COPD. Chest [Internet]. 2023 Nov [cited 2025 Jul 9];164(5). Available from: https://pubmed.ncbi.nlm.nih.gov/37690008/
-
[6]
Health Quality Ontario (HQO). Asthma in Adults: The Quality Standard in Brief [Internet]. 2025 [cited 2025 Jul 9]. Available from: https://www.hqontario.ca/Evidence-to-Improve-Care/Quality-Standards/View-all-Quality-Standards/Asthma-in-Adults/The-Quality-Standard-in-Brief
-
[7]
Chronic Obstructive Pulmonary Disease (COPD) – Health Quality Ontario (HQO) [Internet]. 2023 [cited 2025 Jul 9]. Available from: https://www.hqontario.ca/Evidence-to-Improve-Care/Quality-Standards/View-all-Quality-Standards/Chronic-Obstructive-Pulmonary-Disease
-
[8]
Agustí A, Celli BR, Criner GJ, Halpin D, Anzueto A, Barnes P, et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Am J Respir Crit Care Med. 2023 Mar 1;207(7):819.
-
[9]
US Department of Veterans Affairs. Management of Chronic Obstructive Pulmonary Disease (COPD) [Internet]. 2021 [cited 2025 Jul 9]. Available from: https://www.healthquality.va.gov/guidelines/cd/copd/
-
[10]
NICE. Recommendations | Chronic obstructive pulmonary disease in over 16s: diagnosis and management | Guidance | NICE [Internet]. NICE; [cited 2021 Feb 24]. Available from: https://www.nice.org.uk/guidance/ng115/chapter/Recommendations#managing-stable-copd
-
[11]
National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management (BTS, NICE, SIGN) [Internet]. NICE; 2024 [cited 2025 Jul 9]. Available from: https://www.nice.org.uk/guidance/NG245
-
[12]
-
[13]
Asthma Canada. Beat the Heat: 9 Tips for Managing Your Asthma in the Summer [Internet]. 2025. Available from: https://asthma.ca/beat-the-heat-9-tips-for-managing-your-asthma-in-the-summer/
-
[14]
Asthma Canada. Breathe EasyTM: Triggers [Internet]. 2016 [cited 2025 Jul 9]. Available from: https://asthma.ca/breathe-easy-triggers/
-
[15]
American Lung Association. Prevent a COPD Exacerbation or Flare Up [Internet]. 2025. Available from: https://www.lung.org/lung-health-diseases/lung-disease-lookup/copd/living-with-copd/prevent-flare-ups
-
[16]
Othar L. Managing COPD Year-Round: Weather Tips for COPD Patients [Internet]. Chronic Lung Diseases. 2024 [cited 2025 Jul 9]. Available from: https://chroniclungdiseases.com/en/news/weather-tips-for-copd-patients/
-
[17]
Lung Health Foundation. Living with COPD [Internet]. 2021. Available from: https://lunghealth.ca/wp-content/uploads/2021/06/2021_LHF_COPD-Handbook-2.pdf
-
[18]
Health Quality Ontario (HQO). Heart Failure Quality Standard [Internet]. 2022 [cited 2025 Jul 11]. Available from: https://www.hqontario.ca/evidence-to-improve-care/quality-standards/view-all-quality-standards/heart-failure
-
[19]
Chaseling G, Uchmanowicz I, Bäck M, Miró O, Tokmakova M, Ljungman P, et al. Heat extremes and cardiovascular diseases: a scientific statement of the Association of Cardiovascular Nursing & Allied Professions, Association for Acute Cardiovascular Care, European Association of Preventive Cardiology, Heart Failure Association, European Heart Rhythm Association of the ESC, the ESC Council on Hypertension, the ESC Council on Stroke, and the ESC Working Group on Cardiovascular Pharmacotherapy. Eur Heart J [Internet]. 2025 Mar 6 [cited 2025 Jul 11]; Available from: https://pubmed.ncbi.nlm.nih.gov/40457965/
-
[20]
Bozkurt B, Coats Aj, Tsutsui H, Abdelhamid C, Adamopoulos S, Albert N, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail [Internet]. 2021 Mar [cited 2025 Jul 11];23(3). Available from: https://pubmed.ncbi.nlm.nih.gov/33605000/
-
[21]
Heidenreich P, Bozkurt B, Aguilar D, Allen L, Byun J, Colvin M, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol [Internet]. 2022 Mar 5 [cited 2025 Jul 11];79(17). Available from: https://pubmed.ncbi.nlm.nih.gov/35379503/
-
[22]
Ezekowitz J, O’Meara E, McDonald M, Abrams H, Chan M, Ducharme A, et al. 2017 Comprehensive Update of the Canadian Cardiovascular Society Guidelines for the Management of Heart Failure. Can J Cardiol [Internet]. 2017 Nov [cited 2025 Jul 11];33(11). Available from: https://pubmed.ncbi.nlm.nih.gov/29111106/
-
[23]
British Heart Foundation. How cold weather affects your heart [Internet]. 2024. Available from: https://www.bhf.org.uk/informationsupport/support/practical-support/will-cold-weather-affect-my-heart-condition#h3
-
[24]
Dedegikas C. Winter with heart failure: Things to be mindful of this season – HeartLife Foundation [Internet]. 2018 [cited 2025 Jul 11]. Available from: https://heartlife.ca/patients/winter-with-heart-failure-things-to-be-mindful-of-this-season/
-
[25]
Ontario College of Family Physicians. Family Physician Respiratory Illness Season Resource Checklist [Internet]. 2023. Available from: https://www.ontariofamilyphysicians.ca/wp-content/uploads/2023/10/ocfp_fp_respiratoryillnessseasonchecklist_2023_final-1.pdf
-
[26]
Public Health Ontario. Best Practices for the Prevention of Acute Respiratory Infection Transmission in All Health Care Settings [Internet]. 2025. Available from: https://www.publichealthontario.ca/-/media/Documents/A/24/acute-respiratory-infection-transmission.pdf?rev=6ce1a644c24d41f982b3a2b3736eb3fd&sc_lang=en&hash=307085FD484276386DE55EC9EA0A3C0C
-
[27]
Public Health Ontario. Building Confidence in Vaccines [Internet]. 2021. Available from: https://www.publichealthontario.ca/-/media/documents/ncov/vaccines/2021/04/covid-19-building-confidence-in-vaccines.pdf?la=en
-
[28]
Public Health Agency of Canada. Canadian Immunization Guide [Internet]. 2024. Available from: https://www.canada.ca/en/public-health/services/canadian-immunization-guide.html
-
[29]
Public Health Agency of Canada. Immunization Partnership Fund [Internet]. 2025. Available from: https://www.canada.ca/en/public-health/services/immunization-vaccine-priorities/immunization-partnership-fund.html
-
[30]
Lyons R, Mathews M, Ryan D, Hedden L, Lukewich J, Marshall EG, et al. A Qualitative Analysis of the Functions of Primary Care Nurses in COVID‐19 Vaccination. J Adv Nurs. 2024 Sept 20;81(5):2510.
-
[31]
Stratoberdha D, Gobis B, Ziemczonek A, Yuen J, Giang A, Zed P. Barriers to adult vaccination in Canada: A qualitative systematic review. Can Pharm J CPJ Rev Pharm Can RPC. 2022 June 6;155(4):206–18.
-
[32]
Ontario Ministry of Health. Health Care Provider Fact Sheet: Pneumococcal Conjugate Vaccines for Adults Aged 18 Years and Older [Internet]. 2025. Available from: https://www.ontario.ca/files/2025-07/moh-hcp-fact-sheet-pneumococcal-vaccine-18-and-older-en-2025-07-01.pdf
-
[33]
Public Health Agency of Canada. Pneumococcal vaccines: Canadian Immunization Guide: For health professionals [Internet]. 2025. Available from: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-16-pneumococcal-vaccine.html#a18
-
[34]
Ontario Ministry of Health. Health Care Provider Fact Sheet: Influenza Immunization for Individuals 6 months to 64 years of age [Internet]. 2025. Available from: https://www.ontario.ca/files/2025–09/moh-uiip-25-26-6mo-64-fact-sheet-en-2024-09-10.pdf
-
[35]
Ontario Ministry of Health. Health Care Provider Fact Sheet: Influenza Immunization for Individuals ≥65 years of age [Internet]. 2025. Available from: https://www.ontario.ca/files/2025–09/moh-uiip-25-26-65-plus-fact-sheet-en-2025–09-10.pdf
-
[36]
Ontario Health. Recommendations for Antiviral Therapy of Seasonal Influenza [Internet]. 2025. Available from: https://www.ontariohealth.ca/content/dam/ontariohealth/documents/recommendations-antiviral-therapy-seasonal-influenza.pdf
-
[37]
Ontario Ministry of Health. Health Care Provider Fact Sheet: 2025/2026 COVID-19 Vaccine Program [Internet]. 2025. Available from: https://www.ontario.ca/files/2025–09/moh-covid-vaccine-hcp-fact-sheet-en-2025–09-12.pdf
-
[38]
Ontario Ministry of Health. COVID‑19 testing and treatment [Internet]. 2025 [cited 2025 Oct 6]. Available from: http://www.ontario.ca/page/covid-19-testing-and-treatment
-
[39]
Public Health Agency of Canada. Respiratory syncytial virus (RSV) vaccines: Canadian Immunization Guide [Internet]. 2025 [cited 2025 Aug 7]. Available from: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/respiratory-syncytial-virus.html
-
[40]
Public Health Agency of Canada. Summary of NACI statement of March 13, 2025: Updated guidance on respiratory syncytial virus (RSV) vaccines for older adults including the expanded use of RSVPreF3 for individuals 50 to 59 years of age and use of the new mRNA-1345 vaccine [Internet]. 2025. Available from: https://www.canada.ca/en/public-health/services/publications/vaccines-immunization/national-advisory-committee-immunization-summary-updated-guidance-rsv-vaccines-older-adults-including-expanded-use-rsvpref3-individuals-50-59-years-age-use-new-mrna-1345-vaccine.html
-
[41]
Ontario Ministry of Health. Respiratory Syncytial Virus [Internet]. 2025. Available from: https://www.ontario.ca/page/respiratory-syncytial-virus#:~:text=2023%20RSV%20season-,Through%20the%20Respiratory%20Syncytial%20Virus%20Prophylaxis%20for%20High-Risk%20Infants,2%20years%20of%20age%20at
-
[42]
Ontario Ministry of Health. Older Adult High-Risk Respiratory Syncytial Virus (RSV) Vaccine Program Fact Sheet – Health Care Providers [Internet]. 2025. Available from: https://www.wechu.org/sites/default/files/HSch%3B%20IMMS%20-%20Correspondence%20-%20Older%20Adult%20High%20Risk%20RSV%20Vaccine%20Program%20Fact%20sheet%20for%20HCP-%20ENG%20Aug%20%28ID%20315550%29.pdf
-
[43]
Expert opinion, Chief Medical Officer of Health.
-
[44]
Ontario Ministry of Health. Respiratory Syncytial Virus (RSV) prevention programs [Internet]. 2025. Available from: https://www.ontario.ca/page/respiratory-syncytial-virus-rsv-prevention-programs#section-2
-
[45]
Ontario Ministry of Health. Bulletin 251001 — New diagnostic code for Respiratory Syncytial Virus (RSV) | OHIP INFOBulletins 2025 [Internet]. 2025 [cited 2025 Oct 6]. Available from: http://www.ontario.ca/document/ohip-infobulletins-2025/bulletin-251001-new-diagnostic-code-respiratory-syncytial-virus
-
[46]
Ontario Ministry of Health. Infant and High-risk Children Respiratory Syncytial Virus (RSV) Prevention Program Factsheet for Health Care Providers [Internet]. 2025. Available from: https://www.ontario.ca/files/2025–09/moh-infant-high-risk-children-rsv-guidance-hcp-en-2025–09-04_0.pdf
-
[47]
Public Health Ontario. Adverse Event Following Immunization Reporting for Health Care Providers in Ontario [Internet]. 2025. Available from: https://www.publichealthontario.ca/-/media/Documents/A/2016/aefi-reporting-overview.pdf?rev=724a344bf81a4ad28e74cb4985975558&sc_lang=en&hash=4C4BC7A0C122066CD1AFA4BFD0CB9682
-
[48]
Public Health Agency of Canada. Storage and handling of immunizing agents: Canadian Immunization Guide [Internet]. 2022. Available from: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-1-key-immunization-information/page-9-storage-handling-immunizing-agents.html
-
[49]
Public Health Agency of Canada. Vaccine supply [Internet]. 2024 [cited 2025 Sept 19]. Available from: https://www.canada.ca/en/public-health/services/vaccine-supply.html
-
[50]
Public Health Agency of Canada. Communicating effectively about immunization: Canadian Immunization Guide [Internet]. 2025. Available from: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-1-key-immunization-information/page-5-communicating-effectively-immunization.html
-
[51]
Public Health Agency of Canada. Community-based projects help expand access to vaccinations and credible information on immunization [Internet]. 2023. Available from: https://www.canada.ca/en/public-health/news/2023/12/community-based-projects-help-expand-access-to-vaccinations-and-credible-information-on-immunization.html
-
[52]
Health Canada. Common Definitions on Cultural Safety: Chief Public Health Officer Health Professional Forum [Internet]. 2023. Available from: https://www.canada.ca/en/health-canada/services/publications/health-system-services/chief-public-health-officer-health-professional-forum-common-definitions-cultural-safety.html
-
[53]
Public Health Agency of Canada. Full report: Realizing the Future of Vaccination for Public Health: The Chief Public Health Officer of Canada’s Report on the State of Public Health in Canada 2024 [Internet]. 2024. Available from: https://www.canada.ca/en/public-health/corporate/publications/chief-public-health-officer-reports-state-public-health-canada/state-public-health-canada-2024/report.html#a3
-
[54]
National Collaborating Centre for Indigenous Health. Vaccine Uptake among First Nations, Inuit, and Metis Populations [Internet]. 2024. Available from: https://www.nccih.ca/Publications/Lists/Publications/Attachments/10468/FS-vaccine-uptake-EN-Web.pdf
-
[55]
Health System Emergency Management Branch, Ministry of Health. Seasonal Respiratory Pathogen Guide Version 2.0 [Internet]. 2025. Available from: https://www.ontario.ca/files/2025-07/moh-seasonal-respiratory-pathogen-guide-en-2025-07-21.pdf
-
[56]
Ontario Medical Association. Flu vaccine [Internet]. 2025. Available from: https://www.oma.org/practice-professional-support/running-your-practice/patient-care/vaccines-physicians-rights-and-responsibilities/flu-vaccine/#:~:text=years%20of%20age-,Billing,-Billing%20codes%20for
-
[57]
Ontario Medical Association. RSV prevention programs [Internet]. 2025. Available from: https://www.oma.org/practice-professional-support/running-your-practice/patient-care/vaccines-physicians-rights-and-responsibilities/rsv-immunization-program/