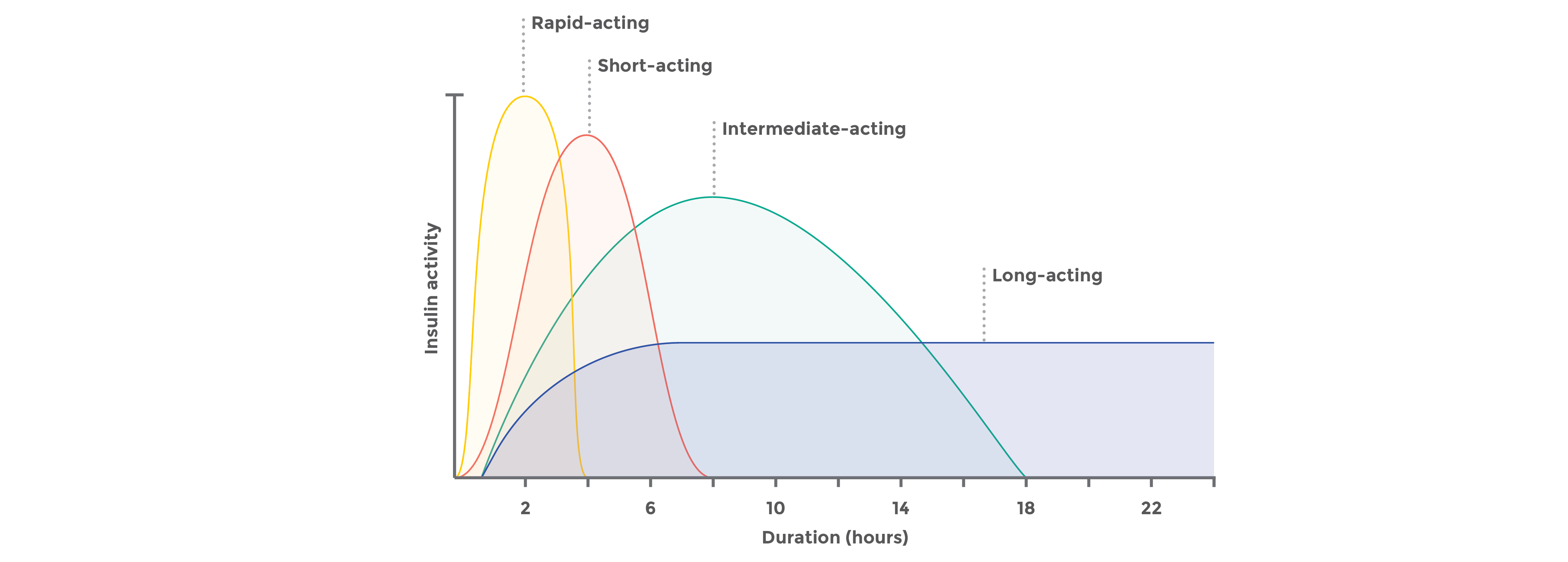
Biosimilar insulins33-42
As of March 31, 2023, patients who are eligible for ODB program benefits and using insulin lispro (Humalog), insulin aspart (NovoRapid), and insulin glargine (Lantus) are required to transition to a biosimilar. This is a 1:1 – same dose switch.
A biosimilar is a biologic drug that is highly similar to a biologic drug that was already authorized for sale. At a reduced cost, biosimilars have equivalent clinical efficacy and safety as their originator biologic drug.
Originator biologic insulins listed above will not be covered through the ODB as of December 29, 2023, unless patients have been approved for a medically necessary exemption. To avoid gaps in coverage, during the 9-month transition period that ends in December 2023, ODB will cover both the originator and biosimilar options for patients currently receiving insulin therapy. The transition period will provide time for health care providers to become informed and subsequently have discussions with patients about biosimilar transition. This transition period will also allow time for prescribers to write a new prescription and provide time for patients to obtain their new insulin and, in some-cases, new insulin pens from their pharmacy.
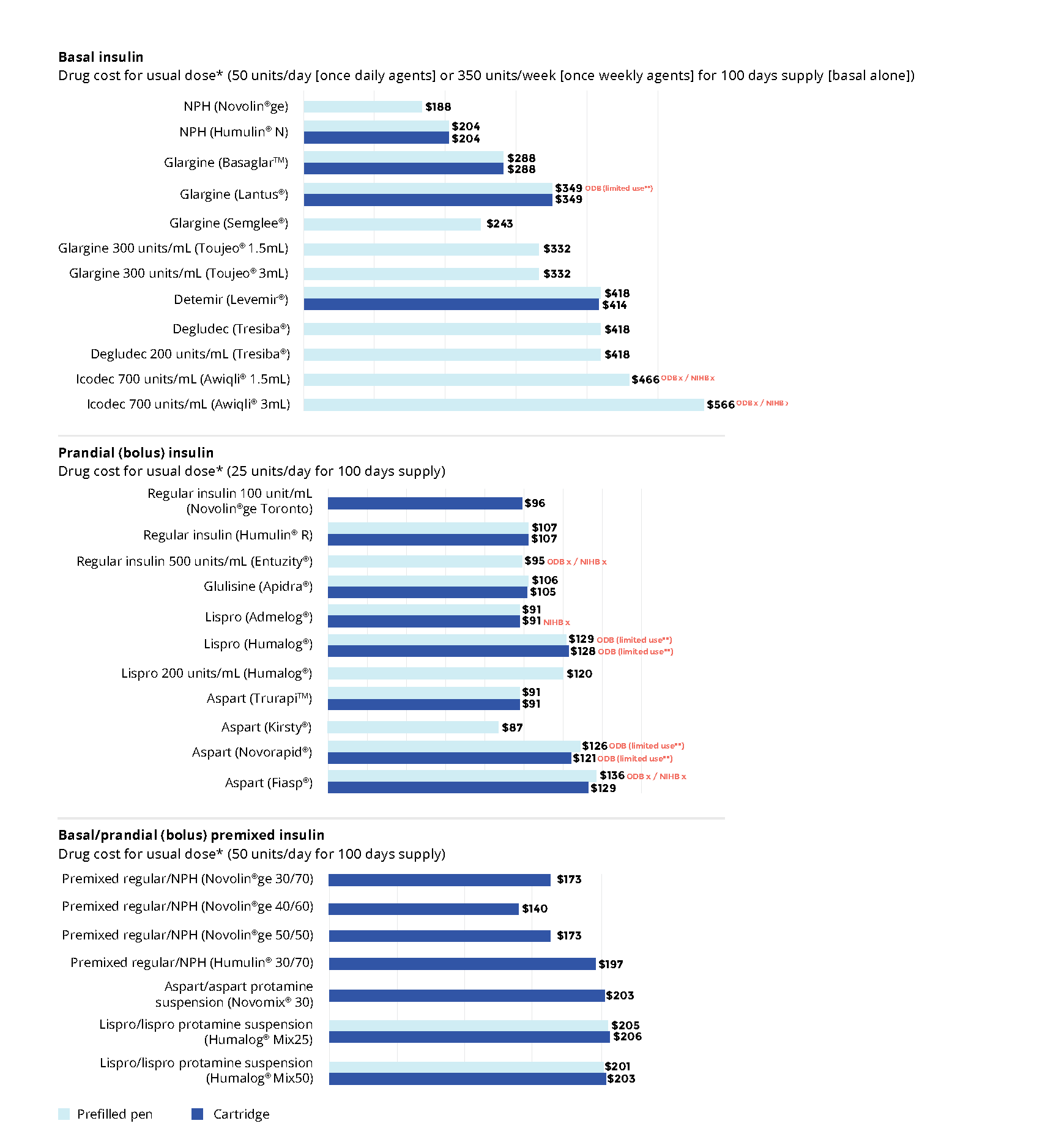
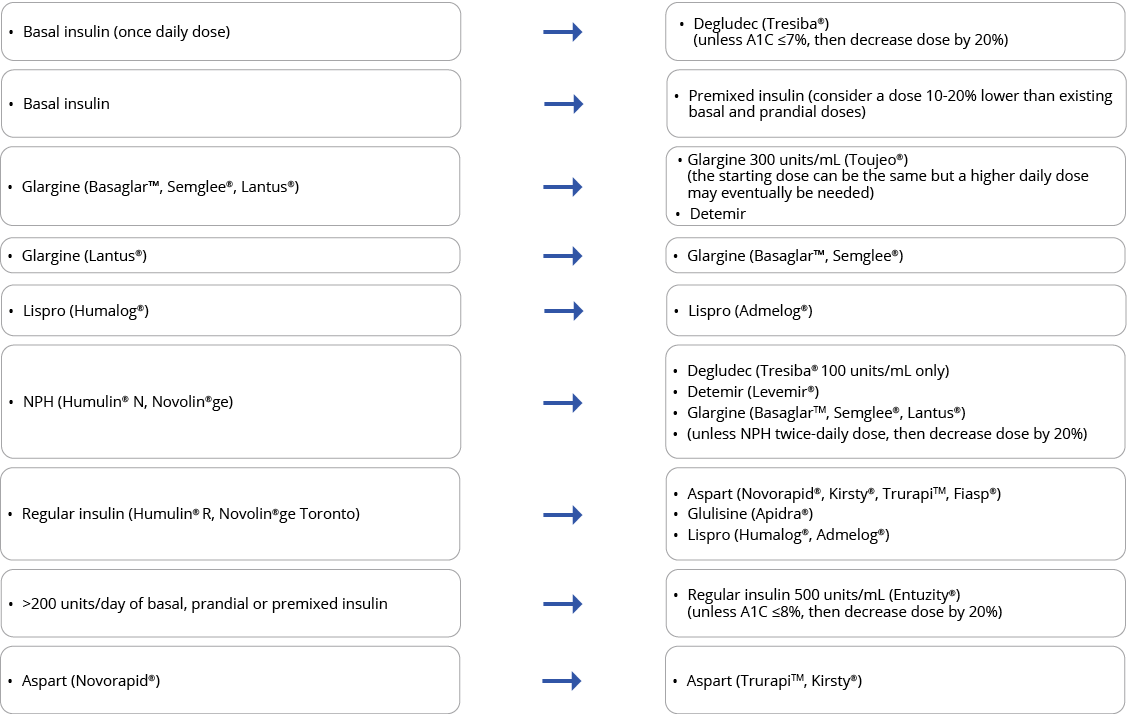
Below are the biosimilar insulins that are available in Ontario:
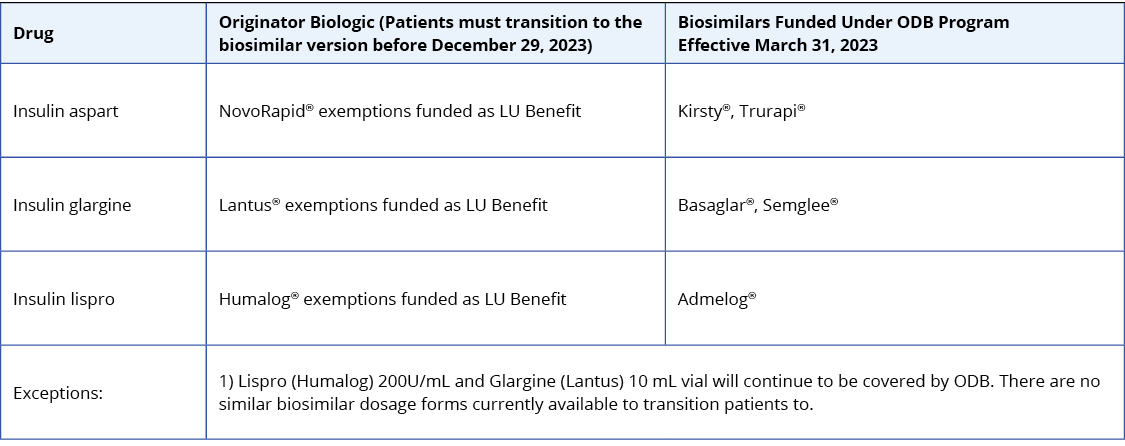
For insulin-naive patients, consider biosimilar insulins in the table above as the first treatment option given their cost-effective advantage.
For patients on an established insulin therapy and requiring a switch to a biosimilar, consider the following approach. Submit a claim for K900A when switching patients from an originator biologic insulin to a biosimilar to receive a support payment of $61.20.
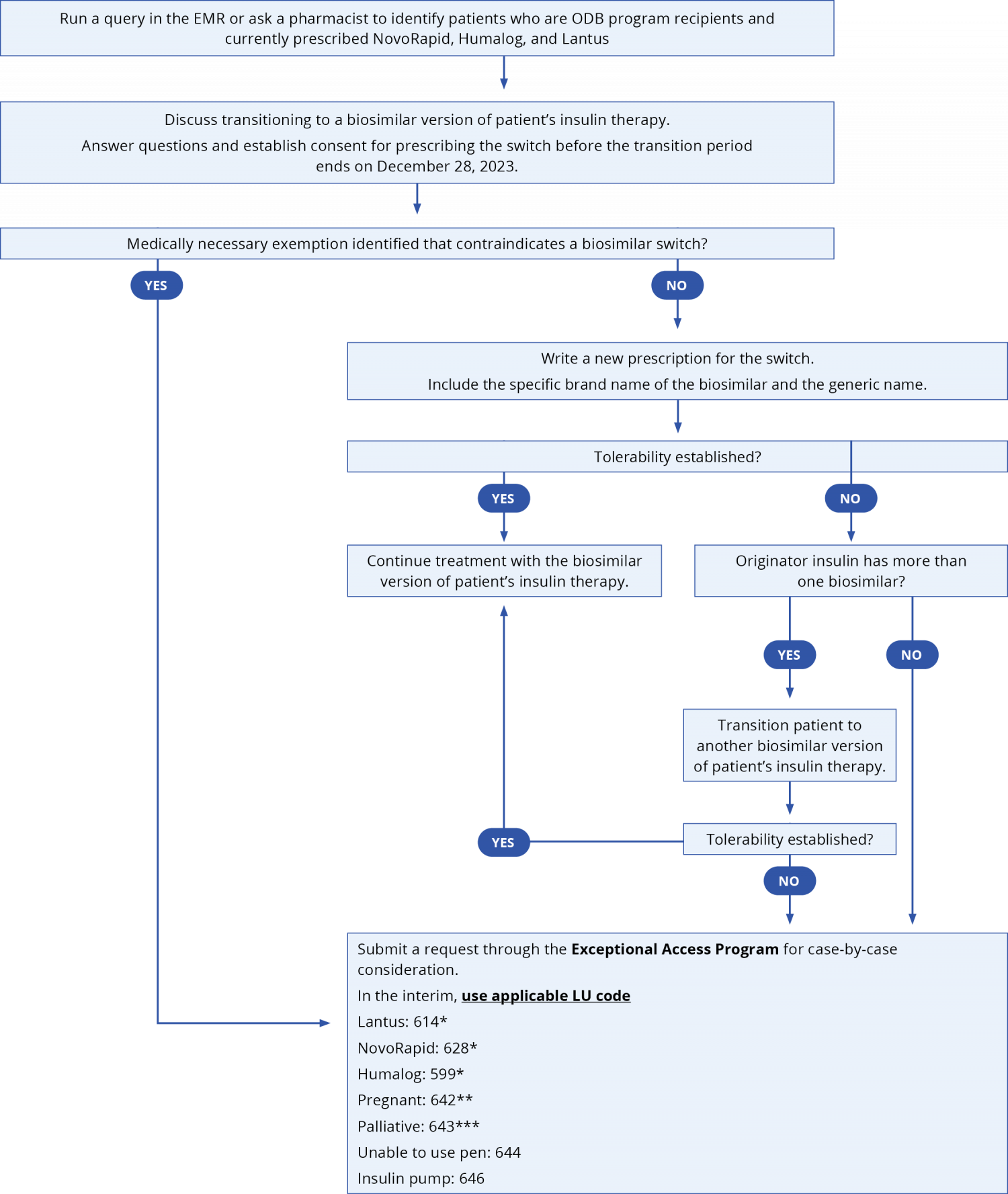
*For patients awaiting medically necessary exemption via the EAP that were already established on originator insulins therapy before March 2023; **For patients established on therapy prior to March 31, 2023 who is or has become pregnant during the biosimilar transition period between March 31, 2023 to December 28, 2023; ***For patients established on therapy prior to March 31, 2023 who is or becomes palliative requiring end-of-life care during the biosimilar transition period between March 31, 2023 to December 28, 2023.
Click here for EMR queries to identify patients who are ODB program recipients and currently prescribed NovoRapid, Humalog, and Lantus.
The patient’s new biosimilar insulin pen(s) may look different from the originator insulin. Encourage patients to consult their pharmacist to minimize confusion about the change in appearance, especially if they use different pens for basal and bolus therapy. If switching both basal and bolus therapy to biosimilars, consider temporally separate switches to reduce medication errors.
Medically necessary exemptions
To ensure no gaps in patient coverage through the ODB, submit EAP requests promptly through the Special Authorization Digital Information Exchange. Requests can also be faxed using the EAP form to 1-866-811-9908 (toll-free) or 416-327-7526 (Toronto area)