This tool is designed to help providers understand, assess, and manage patients/residents in primary care and LTC homes with behavioural and psychological symptoms of dementia (responsive behaviours), with a focus on antipsychotic medications. It was developed as part of Centre for Effective Practice’s Academic Detailing Service for LTC homes. This tool integrates best-practice evidence with clinical experience, and makes reference to relevant existing tools and services wherever possible.
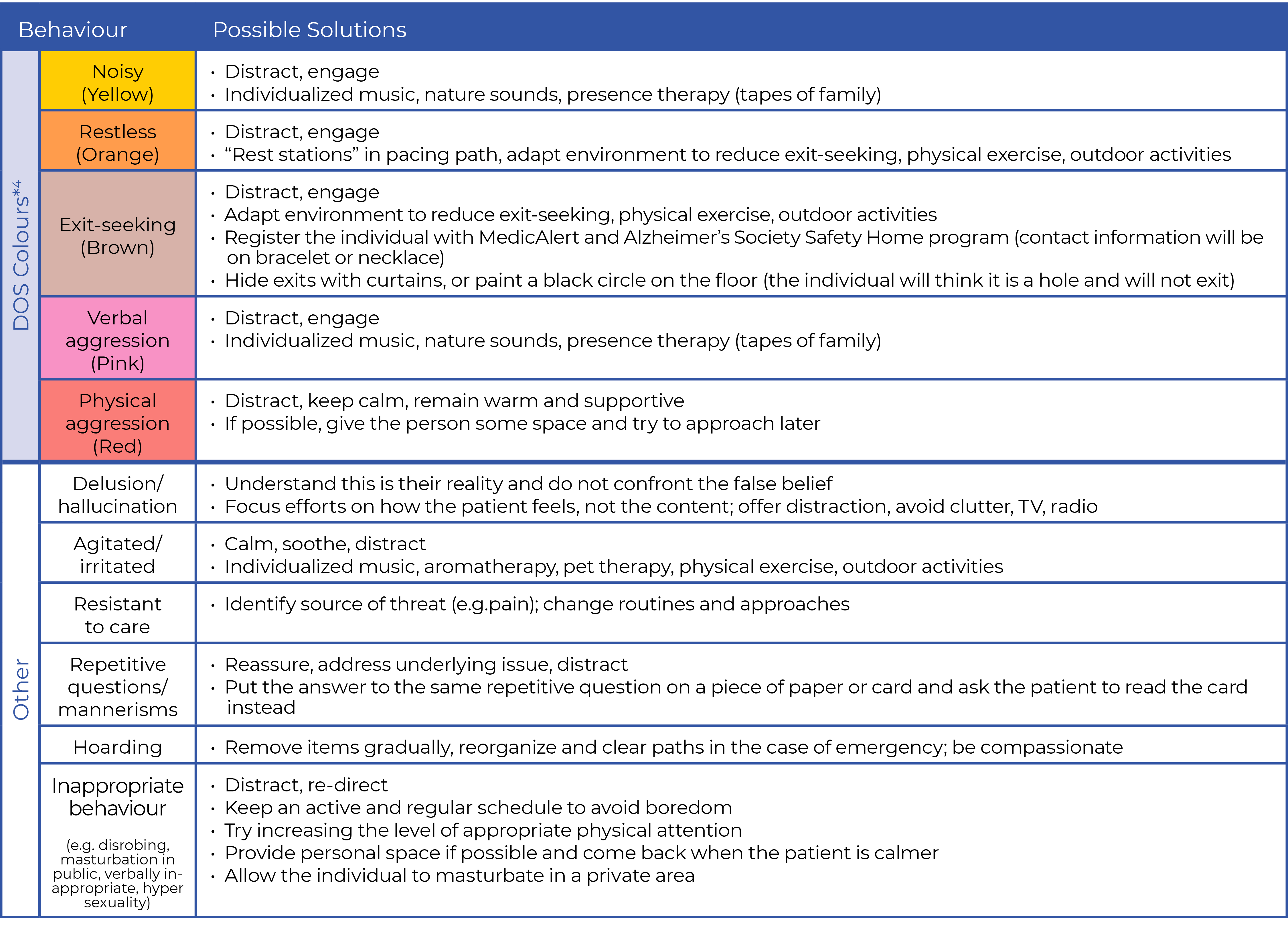
Talking points: Use P.I.E.C.E.S.TM to identify risks and BPSD causes
Use the P.I.E.C.E.S. 3-Question Template TM to ask:
- What has changed?
- What are the RISKS and possible causes?
- What is the action?
Physical
Think “the 5 Ds”
- Delirium
- Disease (cardiovascular, infectious, insomnia, metabolic, nocturia, renal, respiratory, sleep apnea, urinary retention, etc)
- Drugs (e.g. acetycholinesterase inhibitors, anticholinergics, anticonvulsants, anti-Parkinson, benzodiazepines, digoxin, fluoroquinolones, lithium, opioids, systemic corticosteroid) See Reference List of Drugs with Anticholinergic Effects41
- Discomfort (e.g. pain, constipation, fecal impaction, urinary retention, hunger, thirst)
- Disability (e.g. sensory loss)
Intellectual
Think “the 7 As”
- Amnesia (memory)
- Aphasia (speech)
- Apathy (initiative)
- Agnosia (recognition of people or things)
- Apraxia (purposeful movement)
- Anosognosia (insight/self-awareness)
- Altered Perception (sensory information)
Emotional
Think “the 4 Ds”
- Disorder Adjustment (e.g. related to losses)
- Disorders of Mood (e.g. depressive symptoms, anxiety)
- Delusional (e.g. suspiciousness, psychosis)
- Disorders of Personality
Capabilities
- Capability too low to meet demands of environment (catastrophic reactions) or not utilized enough (boredom)
- Maximize remaining strengths; avoid unnecessary disability
Environment
- Consider over-/under-stimulation, relocation, change in routine, noise, lighting, colours, social interactions with caregivers/others
Social
- Consider social network, life story, cultural/spiritual heritage